When winter rolls around you may notice trucks spreading salt all over the road. The salt melts the ice making the roads grippier and safer, but have you ever thought about the science behind this? Why exactly does salt melt ice and how does it work?
Salt melt ice because salt molecules get between the water molecules preventing them from solidifying into ice at 0ºC (32ºF). The now salty water requires a lower temperature of -9ºC (16ºF) to remain as ice otherwise it will break down into a liquid. This process is called freezing point depression.
Salt Lowers The Freezing Point Of Water (Freezing Point Depression)

Effectively what happens when you add salt to ice is that the salt molecules dissolve into the water and cause what is known as “freezing point depression”.
What this means is that it now takes a lower temperature in order for the salt water solution to turn into ice, or remain in its solid ice state.
For example we know that water freezes and turns into ice at 0ºC (32ºF). It changes from a liquid into a solid.
We also know that once the temperature rises above 0ºC (32ºF) the ice begins to melt and turn from a solid back into it's liquid form.

When salt is added to ice and freezing point depression occurs the new freezing temperature is lowered. How low it goes depends on the particular salt used but regular table salt (sodium chloride) can depress the freezing point of water as low as -9ºC (16ºF).
What this means is that the temperature needs to go down to at least -9ºC (16ºF) in order for this salty water to turn into solid ice. It also means that if temperatures rise above -9ºC (16ºF) then the solid ice will begin to melt and turn back into it's liquid form.
This freezing point depression means that ice with salt added to it will begin to melt even in temperatures below 0ºC (32ºF).
For example, let's say the outside temperature is -5ºC (23ºF). This is cold enough that regular water will turn into ice and stay as ice.
However, this temperature is ABOVE the -9ºC (16ºF) required to turn salt water into ice. This means the salted ice will begin to melt because temperatures are not cold enough for it to remain in its solid state.
There are other salts that after more effective at freezing point depression and can cause ice to melt in temperatures as low as -20ºC (-4ºF) or even as low as -70ºC (-94ºF). However, these tend to be more expensive.
How Exactly Does Salt Melt Ice Molecularly? Step-By-Step Explanation
To understand how salt melts ice it's a good idea to understand the entire process step-by-step on a molecular level.
Once you understand this process it becomes quite clear how exactly the salt works to melt ice even at temperatures at or below 0ºC (32ºF).
1. Regular Water Forms Into a Crystal Like Lattice To Form Ice
For salt to melt ice we first need to have ice in the first place. Understanding how the ice forms is vital to understand how the ice interrupts this process and causes the structure of ice to break down.
At 0ºC (32ºF) water will begin to move from it's free flowing liquid form and will begin to form into hexagonal crystalline structures (wikipedia)
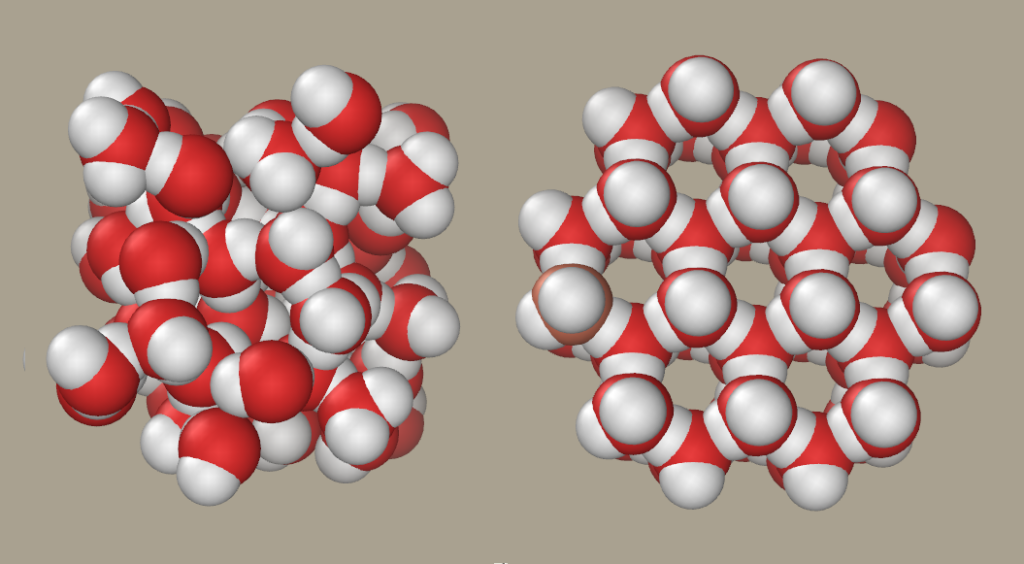
You can see in the diagram above that the molecular structure of the water changes and the H2O change from being more free flowing and randomly organized to forming a hexagonal structure that gives ice its solid shape.
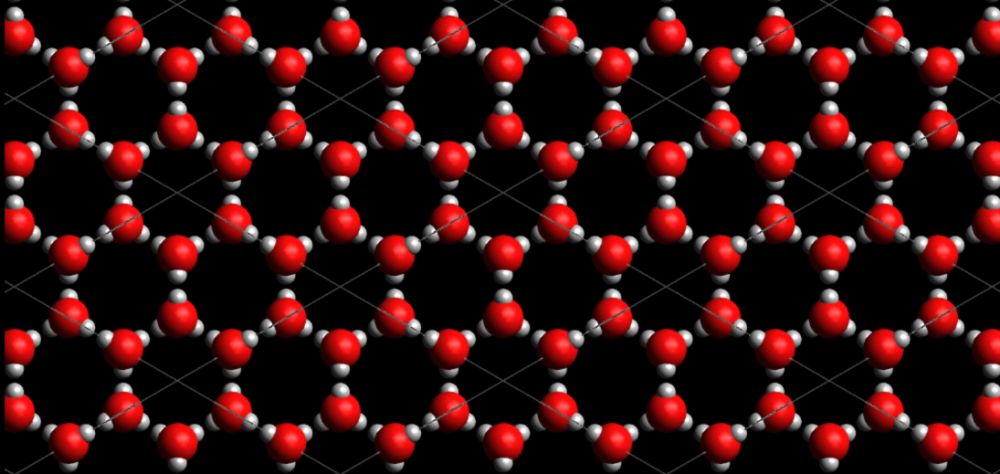
The oxygen atoms in the water are positively charged while the hydrogen atoms are negatively charged. This causes them to attract to each other and with the help of hydrogen bonds form into ice crystals and ultimately ice blocks. (Salt on the other hand is held together by ionic bonds)
2. A Thin Layer Of Water Exists On Top Of The Ice

You may not realize this looking at the ice of the road or the sidewalk but on top of the ice is a very thin layer of water that exists in equilibrium with the ice.
Some of this thin layer of water will refreeze and turn back into ice while some of the ice will melt and turn into water. This goes on constantly and this thin layer of water is part of what makes the ice so slippery and dangerous to walk or drive on.
The colder it is the thinner this layer will be, but you'll see that this layer of water is extremely important in how the salt melts the ice.
3. The Salt Dissolves Into The Thin Layer Of Water On Top Of The Ice

When the salt is placed on top of the ice (like when a truck spreads it across the road) the salt will immediately begin to dissolve into the ice.
Table salt is made up of equal parts Sodium and Chloride and when they mix with water they break away from their crystalline structure and attach themselves to the water molecules as seen below:
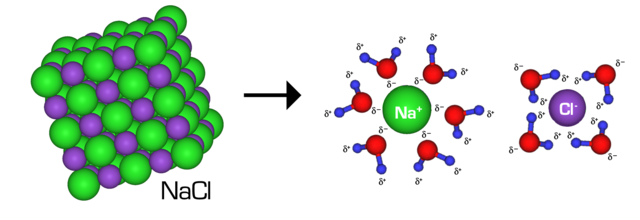
Interestingly, this act of the salt dissolving in the thin layer of water makes that water COLDER as energy in the form of heat is required for this dissolving process to occur.
So the salt doesn't melt the ice because it warms it up. No, salt actually makes ice colder.
So why does salt melt ice then?
4. The Salt Molecules Interrupts Water's Ability To Form The Crystalline Structure Required For Ice – Freezing Point Depression
Now that the salt has dissolved into the water the Sodium and Chloride (or the specific molecules used into other types of ice melt) connect with the H2O molecules in water.
Because of this connection the salt molecules actually get in the way and stop the water molecules from bonding together and forming the hexagonal structure needed into order to turn into ice at 0ºC (32ºF).
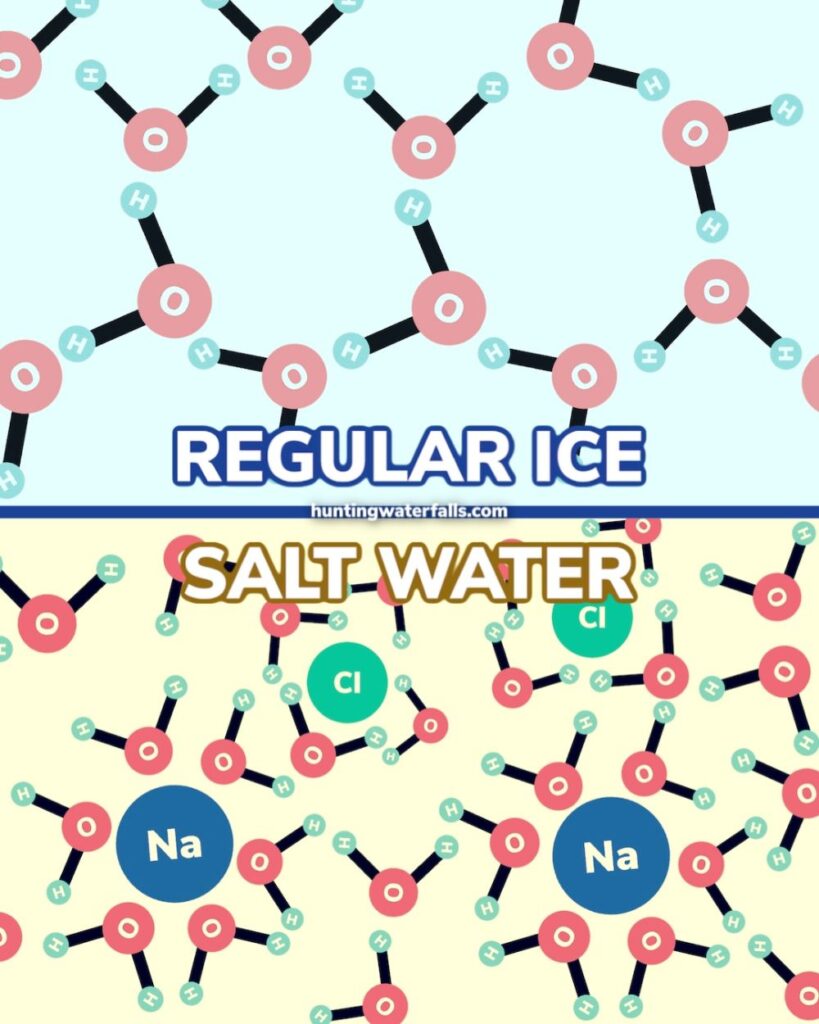
This means at 0ºC (32ºF) the now salty water wants to remain in a liquid form. So that thin layer of now salty water on top of the ice won't refreeze and instead a new layer of water will form below it.
This new layer of water will then mix with the salt and salt solution above it continuing the process. It now will want to stay and liquid and so a new layer of water will form below it and so on.


The water can overcome this interruption from the salt and turn into ice however, a much lower temperature is needed for this to happen.
With regular table salt this temperature is around -9ºC (16ºF). So if air temperatures are below this the salt will not melt the ice and other alternatives to salt for melting ice are needed. Some of these can work in temperatures as low as -20ºC (-4ºF) or even -70ºC (-94ºF) but they are much more expensive and have their own downsides.
5. More Surface Area Is Exposed, Helping The Remaining Ice To Melt Faster

A tried and true method to help ice melt quicker is to break it up. This is why many people will take shovels to their sidewalk. They break the ice off into smaller pieces where it will naturally melt.
Salt does this also. It'll naturally melt the ice it comes in direct contact with, leaving over leftover ice that is hasn't touched or dissolved into.
This leftover ice will have a greater surface area due to the ice melting around it and thus it'll begin to melt faster than it would if it was just one giant sheet or block of ice.
6. Leftover Salt Can Prevent Ice From Reforming
Another benefit of using salt to melt ice on roads and driveways is that the leftover salt will mix with any water or dew that begins to form on the roads or pavements at night and can actually stop the ice from forming in the first place.
A vinegar spray can be used on your car windscreen at night for a similar effect to stop the ice from forming. Vinegar doesn't work as well at stopping ice formation when compared to salt but it's generally considered safer to use on your car than salt due to its corrosion properties.
Videos On Why Salt Melts Ice:
To expand your learning I found the following videos very helpful in demonstrating the science behind why salt melts ice:
If you're still interested in learning more read here about why salt lowers the freezing point of ice.
Most Effective Salts For Melting Ice

If you live in a cold area that gets icy roads chances are you may see a salt truck driving through your area pouring salt or a salted water mixture onto the roads and sidewalks to get the ice to melt.
But what exact type of road salt is that salt truck using to melt the icy road and if you want to buy some salt for your driveway or footpath what are the best salts for the job.
Below you can see a table of the most effective salts for melting ice. If you're in extremely cold climates with colder temperatures then some salts won't work and if you're in warmer climates the more effective salts aren't really worth the extra cost:
| Type Of Ice Melt | Melts Ice To ºC | Melts Ice To ºF |
|---|---|---|
| Sugar | -.1.1ºC | 30ºF |
| Sodium Chloride (Table Salt) | -9ºC | 16ºF |
| Magnesium Chloride | -20ºC | -5ºF |
| Potassium Chloride | -25ºC | -13ºF |
| Calcium Chloride | -31.7ºC | -25ºF |
| Sand | N/A | N/A |
Different areas will use different salts based on their temperatures as well as which salts are the cheapest and most cost effective. When you're using tons of salt per year then the differences in price add up.
Sometimes a brine solution is used to spray on the roads (this can even be mixed with beet juice in low temperatures) and sometimes solid salt is used. Different ice melters work best in different situations and occasions.
You'll notice that sand doesn't have a temperature figure next to it. This is because sand is not sprayed on the road to melt the ice but rather to provide some natural traction on the slippery ice and avoid cars and people from slipping over.
It's generally seen as more environmentally friendly than using salt but it will not actually cause the ice to melt.
Why Is Salt Used On Icy Roads and Not Sugar?
While sugar can also cause freezing point depression and can help ice to melt it is not nearly as effective as salt when it comes to melting ice.
Salt can lower the freezing point of water by approximately 1-2ºC (2-4ºF) whereas regular salt can lower the freezing point of water by 9ºC (16ºF) making it much more effective at melting ice, especially in colder climates.
The excess salt can be bad for plant and marine life and can wash downstream causing salt brine to end up in lakes and rivers.
What Temperature Does Salt Melt Ice?
Salt will melt ice at any temperature above -9ºC (16ºF) and the higher the temperature the more effective it will be. The more salt you use the more effective it will be but at temperatures below -9ºC (16ºF) you'll need to use a different salt than sodium chloride.
Magnesium chloride, Potassium chloride and Calcium chloride are all popular salts to use in extremely cold temperatures.
Why Does Salt Melt Ice Faster Than Sugar?
Yes, salt will melt ice faster than sugar due to the fact it has a greater freezing point depression effective on ice when compared to sugar.
Salt can cause ice to melt as low as -9ºC (16ºC) whereas sugar will only cause ice to melt as low as -2ºC (28ºF).
Does Salt Stop Ice From Forming?
Yes, salt can stop ice from forming in the first place. If salt is placed on a road or pavement then as snow melts or the air condensates it will mix with the salt forming falt water. This salt water solution will need lower temperatures to freeze and so can stop ice from forming in the first place.
False Myths About Why Salt Melts Ice
There are a couple of different myths out there about why salt melts ice that are just completely incorrect. Here we are going to debunk these myths and explain exactly what's happening.
1. The Salt Warms Up The Ice
Some people believe that as the salt mixes with and dissolves in the ice it actually warms up the water/ice and this is what causes it to melt.
The opposite is actually true. Adding salt to ice or water will actually cause the temperature of the ice and water to go down. This is why salt is used to make ice cream.
In the below video you can see that when salt is dissolved into water the temperature actually goes down by approximately 1.5ºC (2.7ºC)
So as the salt dissolves in the thin layer of water on top of the ice it decreases the temperature of that water. This is because energy (which is taken in the form of heat) is required to break the bonds of the salt and water and dissolve the salt.
Also due to freezing point depression the salt will cause the ice to melt quickly. In order for water to break its bonds and turn from a solid into a liquid it needs energy. It takes this from the heat energy in the ice making the ice colder.
So the salt makes the ice colder not warmer and it melts the ice because the salt interrupts the water's ability to form into a solid.
Click here to read more on why salt makes ice colder
2. Salt Dissolving In Ice Add Energy To Melt The Ice
Some people say that as the salt dissolves into the ice it adds energy into the ice which is what warms it up.
As we already mentioned this isn't true. Dissolving salt into water actually requires energy to break the ionic bonds in the salt. So energy is needed to dissolve the salt which makes the ice colder.
3. Table Salt Is Better Than Rock Salt For Melting Ice

I've seen around the internet some people recommending table salt to melt ice over rock salt. However, rock salt and kosher salt are extremely effective at melting ice…sometimes even moreso than table salt.
The theory is that table salt has a greater surface area and thus dissolves quicker into the ice and melts it faster. But real world results, like the experiment by a school kid below you can clearly see rock salt working extremely effective to melt the ice.
My thinking here is that the coarser rock salt is able to pierce deeper down into the ice faster due to its larger size taking it longer to dissolve. This can cause the ice to have a larger surface area overall and melt faster.
The table salt on the other hand dissolves completely on top.
But really both are equally as effective as each other with only minor differences between the 2 options.




