Whether you're looking at running a science experiment, line the inside of your cooler or just looking for something to keep ice from melting you might consider aluminum foil and whether or not it can keep ice from melting.
Aluminum foil does help keep ice from melting but it's not the most effective insulator and will only extend ice retention slightly. Aluminum foil reflects heat radiation which helps keep ice longer but it conducts heat easily. It is best used to supplement a cooler and make it work better, not used by itself.
In an experiment I ran ice kept inside 1 layer of aluminum foil lasted longer than ice without any insulation and ice kept in 3 layers of aluminum foil laster longer than ice in just a single layer. So the aluminum foil definitely stops ice from melting as quickly.
My Experiment
In the above experiment you can see I took 10 pieces of ice directly from my freezer. These ice cubes were 0ºF (-18ºF) from the freezer.
- I placed 10 pieces of ice in a bowl with no insulation
- I placed 10 pieces of ice and wrapped them in 1 layer of aluminum foil
- I placed 10 pieces of ice and wrapped them in 3 layers of aluminum foil
After 1.5 hours I came back to check the results.

No insulation is on the left, 1 layer of aluminum foil is in the center and 3 layers of aluminum foil is on the right
You can clearly see that even a single layer of aluminum foil stopped the ice from melting as quickly and 3 layers worked even better than 1 layer.
So why does this work?
How Does Aluminum Foil Keep Ice From Melting?
To understand how aluminum foil stops ice from melting you have to understand how heat transfer happens.
Ice melts when heat from the outside is transferred to the ice warming it up and causing it to melt. Heat is transferred in 3 different ways
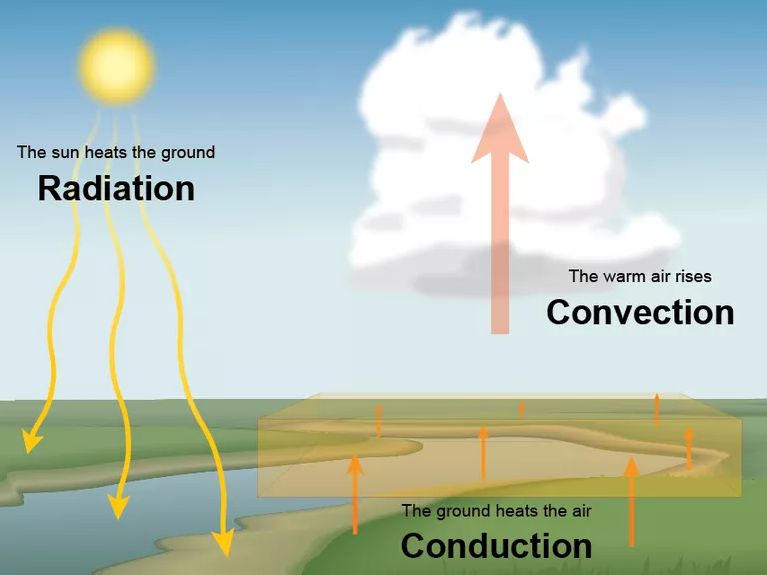
1. Conduction (Moving through a solid object) – Conduction is where heat moves through a solid object or from one object to another. For example placing a pan on a hot stove the heat from the stove moves through the pan making it hot.
2. Convection (Flowing from one place to another) – Convection is when heat flows from one place to another. For example when a fan heater blows hot air around the room or when you put hot water in the bath and push that hot water into a cold section.
3. Heat Radiation – Heat radiation is also know as infrared radiation and this type of heat can move through a vacuum. While the above 2 methods require matter (air, liquid, solid) in order for heat to be transferred heat radiation doesn't require matter.
So how does aluminum foil keep ice from melting by stopping heat transfer?

Aluminum foil reflects 97% of heat radiation and it stops warm air flowing over your ice melting it faster. Multiple layers of aluminum foil trap air pockets which also stop heat conducting through to your ice as easily keeping it frozen for even longer.
Let's break that down…
Aluminum foil is a great reflector of heat radiation. In fact, it reflects up to 97% of heat radiation and is one of the most effective materials for reflecting this type of heat. Copper is also very effective at reflecting heat radiation.
However, being a metal and being super thin aluminum foil is a good conductor of heat. This means heat can pass through the foil really easily. So outside heat can move through the foil to your ice and warm it up fairly quickly.
This makes it bad for keeping ice frozen for a long period of time especially if it is touching something hot as the heat will pass straight through it.
Lastly, aluminum foil is impermeable – meaning air and water can't flow through it. So if you wrap up ice like I did in my experiment no warm air can flow over your ice making it melt faster.
I've got a full article on if aluminum foil is a good insulator for even more details into how aluminum works to trap heat or keep heat out.
What Is The Best Way To Keep Ice From Melting Using Aluminum Foil

While you can use aluminum foil by itself to keep ice from melting it's not the most effective way to use it as the high conductivity of the foil is always going to mean the ice melts fairly quickly.
It's best to use aluminum foil in combination with some other form of insulator that stops heat transfer through conduction.
This is why insulating materials like Reflectix combine aluminum (to reflect heat) and bubble wrap or foam (to stop heat conduction). It's the combination of the 2 types of insulation that works most effectively.
What you want to do is have your ice kept inside an insulating container or cooler and then wrap that container in aluminum foil. Ideally you DO NOT want to aluminum foil to be touching the ice which we will discuss below.
Lining the inside of a cooler with aluminum foil will slightly increase the performance of the cooler because of the reflective qualities of the aluminum foil, but you'll also have the conductivity working against you so the improvements to ice retention will be small.

Lining the outside of a cooler with aluminum foil gives you the benefits of the reflective quality of the aluminum but doesn't give you the conductivity issues you have with aluminum directly touching ice and making it melt more quickly. So overall you'll get a better result.
The cooler stops heat transfer by stopping convection and conduction – it's really good at this. But almost no plastic or styrofoam coolers reflect heat radiation.
Click here to learn how to make a reflectix cooler cover to keep your ice from melting even longer of watch the video below:
By lining your cooler with aluminum foil you'll get the benefits of the cooler as an insulator but you'll also add the reflective properties of the aluminum to reflect away most heat radiation.

If you don't have a cooler then place your ice in a plastic container or wrap the container in aluminum foil and wrap it all in a towel for insulation and your ice will last much longer.
Will Ice Melt Faster On Aluminum or Plastic?
Because aluminum can stop ice from melting you might think that ice would melt slower on aluminum that on other surfaces like plastic or wood, but the opposite is actually true.
Ice will melt much faster on aluminum than plastic. This is because aluminum conducts heat extremely well. The heat of the aluminum block flows quickly into the ice cube melting it while plastic is poor conductor so does not transfer much heat to the ice cube leading it to melt slower.
Below you can see the amazing result and how much faster ice melts on aluminum vs on a plastic foam block.
This can be a really fun experiment to do because when you touch the aluminum it feels cold even at room temperature. This leads you to believe the ice will last longer on the block, whereas in reality the opposite is true
It feels cold because the heat of your hand is rapidly conducted into the aluminum. Even though it's room temperature because the aluminum takes heat from your hand very quickly your brain assumes this means it's cold even when it isn't.
There are a variety of other videos showing very similar results.
The video below compares ice on an aluminum plate to that on a block of wood:
The next video shows how quickly ice melts in a stainless steel bowl vs a plastic bowl.
Stainless steel isn't as conductive as aluminum so the results are less extreme, but it's still obvious that the ice melts a lot faster in the metal bowl than it does in the plastic bowl.
Why Does Ice Melt Faster on Aluminum Than Plastic?
It can be pretty surprising how much fast ice melts on aluminum than it does on plastic. But why does this happen?
Ice melts faster on aluminum than plastic because aluminum is an excellent heat conductor whereas plastic isn't. This means room temperature aluminum quickly transfers heat from itself into the ice cube melting it. Plastic does not transfer much heat at all keeping the ice frozen for longer.
While an aluminum block may feel cold to touch this is just because it conducts heat away from your body so quickly that it tricks your brain and your brain perceives it as cold even though it isn't.
Room temperature aluminum contains a lot of heat (compared to ice) and because this heat can be quickly transferred to the ice the ice will melt extremely quickly.
Room temperature plastic may contain a similar amount of heat but because it's an insulator it's not very good at transferring all that heat into the ice cube so the ice cube will melt slower.




