The first snow day of winter can be charming, but the novelty ends when the ice starts to accumulate and your driveway and paths become slippery and dangerous.
Salt is the most common solution for ice melt but salt can have harmful effects to concrete and some people prefer to use sugar as an alternative ice melt. But which works better and why?
Salt is much more effective at melting ice than sugar as it contains six time more molecules and is better at disrupting the water molecules bonds, causing the ice to melt at lower temperatures. Whereas salt can be effective at melting ice down to around 15ºF/-9ºC, sugar is only effective down to around 28ºF/-2ºC.
Salt and sugar also aren't the only options for melting icy locks and lowering the freezing point of ice and causing it to melt. But they are cheap and readily available solutions.
Let's have a deeper dive into exactly why salt works so well at melting ice whereas sugar is only ok at it as well as some key considerations when creating an ice melt solution this winter.
Why Salt Melts Ice Faster Than Sugar



Adding salt or sugar to ice interrupts the molecular bond that allows the water molecules to transition from a liquid to a solid. This process is called freezing point depression. The freezing point varies depending on the ratio of salt or sugar to water.
What is happening here is that the salt or sugar molecules are getting between the water molecules and making it harder for them to form the bonds required to keep them in solid form.
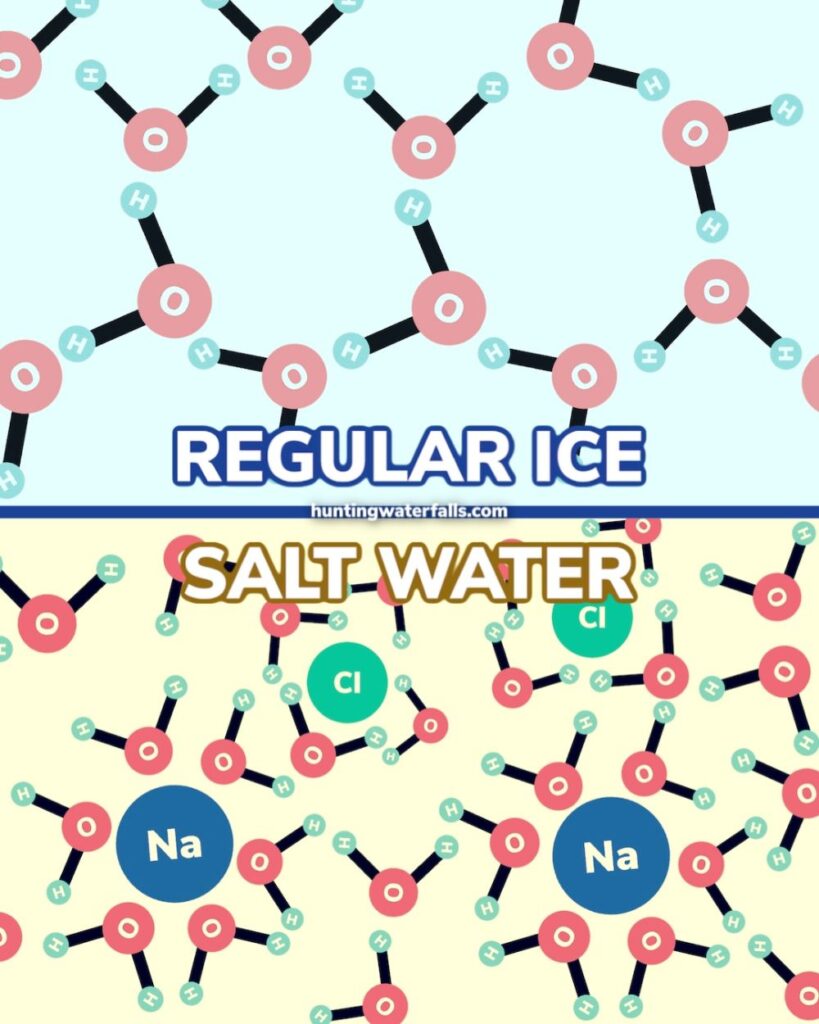
The difference comes down to molecular weight. Chemists use “moles” to measure the amount of substance that is in an object or sample. They then identify the molar mass of a substance which is a ratio to the mass of a substance to its amount of substance.
With salt you need 58.44 grams to make 1 mole, with sugar you need 342.3 grams to make 1 mole. This is a huge difference in molar mass.
The reason salt melts ice faster than sugar is that salt is salt contains more molar mass.
This means you need almost six times the amount of sugar to get the same number of molecules as salt.
In an ice melt solution or direct application, it would take three cups of sugar to have the same effect as 1/2 cup of salt, and that's not even taking minimum working temperatures into account.
Salt and sugar also have minimum working temperatures. Regular table salt (sodium chloride) will work down to around 15ºF/-9ºC before it becomes relatively ineffective at melting ice. For sugar it becomes relatively ineffective at around 28ºF/-2ºC.
So in warmer weather closer to 32ºF/0ºC sugar will work ok (still not as well as salt) but as temperatures get lower sugar will hardly melt ice at all and salt will be needed.
To see a real life example of how sugar and salt melt ice check out the video below:
Why Use Sugar For Ice Melt Instead Of Salt?
If salt works so well for melting ice then why would anyone consider using sugar at all?
Well the reality is that sugar is rarely used to melt ice and salt is almost always more effective and more cost effective too. However, there may be some circumstances where you want to use sugar instead of ice.
The runoff from salt can damage plants whereas the runoff from sugar is safe for plants. So in areas where you don't want your plants to get damaged sugar can be a decent option.
However, something that is plant safe like SafePaw is usually a better option compared to sugar.
This ice melt is 100% salt/chloride free and is made from non-toxic and biodegradable materials. Vet approved, it is safe for pets and children. Fast acting and works down to 10ºF/-12ºC.
Anther time you may want to use sugar is when you are unable to get any salt to melt your ice. Maybe the stores have all sold out or all your salt is used up and all you have left is sugar.
Sugar will melt ice better than nothing.
What Are The Disadvantages of Using Sugar To Melt Ice?

Given sugar is safer for your plants and your concrete you might be wondering why more people don't use sugar to melt ice instead of salt. Doesn't it seem like a good solution?
Well unfortunately there are some major downsides to using sugar to melt ice instead of salt.
The Sugar Makes Your Boots (and Tires) Sticky
Ice melt tends to be used on paths that people are walking or or cars are driving on and if you've ever gotten a sugar mixture all over your hands you likely know how sticky it can get.
If you're using sugar on your path then your boots are going to get very sticky and using it on driveways or roads means your car is going to get a lot of sticky sugar all over it.
Sugar Is Hydroscopic and Holds Onto The Water After The Ice Has Melted
While salt is hydroscopic and holds onto water too sugar does this even more than salt. This means the water will hang around a lot longer if you use sugar when compared to salt and this could lead to more refreezing.
It Takes More Sugar To Melt Ice Compared To Salt
As mentioned earlier salt contains around 6 times more molar mass when compared to sugar. This mean you need 6 grams of sugar for every 1 gram of salt to achieve a similar ice melt result.
This isn't cost effective and also feels excessive when applying the sugar over the ice.
Sugar Only Works at Higher Temperatures
Salt has a lower working temperature than sugar. Sugar is effective at melting ice down to around 28ºF/-2ºC whereas regular table salt is effective down to about 15ºF/-9ºC and other ice melt salts like calcium chloride can work in temperatures as low as -25ºF/-31ºC.
Do Different Salts Melt Ice Faster?

Several types of salt alter the temperature at which ice melts. Rock salt (Sodium chloride) is a popular option for its affordability and instant traction. However, it's not as effective as the inorganic salt Calcium chloride in colder temperatures.
Table salt, kosher salt, or sea salt will also work. They are exactly the same formulation as rock salt ice melts but these options aren't ideal from a cost standpoint as they are more expensive due to the fact they are more tightly regulated as they are for human consumption.
Does Baking Soda Melt Ice Faster than Salt?

Baking soda also lowers the freezing point of water and interrupts the molecular bonds. It can be a good alternative to salt for melting ice in some circumstances however, like sugar, baking soda is less effective than salt, as salt has more molar mass.
Sugar is also more effective than baking soda for melting ice due to its granularity. This option should be a last resort.
Does Salt or Sand Melt Ice Faster?
There's a misconception that sand also melts ice but in reality sand does not melt ice – it just sits on the top of the ice and provides traction for people or vehicles traveling over the ice.
Sand is often used on ice as an eco-friendly way to add traction for cars and pedestrians. While adding sand to water can moderately slow the freezing process, it doesn't interrupt existing molecular bonds.
Downsides of Using Salt to Melt Ice

There are several disadvantages to using salt as an ice melt. Salt is highly corrosive, causing rust and degradation over time.
Rust is a common issue for car owners in icy countries where salt is used on the roads. Many people in the Northern States and Canada purchase used cars from the Southern states for this reason.
Salt can also shorten the lifespan of your concrete steps, walkways, or paved driveway when used throughout the winter. It does this through direct chemical damage, exacerbation of the freeze/thaw cycle and by causing rebar corrosion.
Your pets and plants are also at risk when salt is used. The runoff can get into ditches and waterways, negatively affecting the water supply. Pet owners should be vigilant in ensuring their pets don't lick the salt.
Other Ways to Melt Ice Quickly
If you're looking for an alternative to salt or sugar, try these effective ice melt solutions.
Isopropyl Alcohol
Rubbing alcohol is an effective spot treatment for icy locks and door handles. Isopropyl alcohol functions similarly to salt, causing freezing point depression.
Poor the isopropyl alcohol directly on frozen locks or handles and wait a few minutes to let it soak. You can also mix the alcohol with hot water and dish soap to de-ice windshields and windows before driving or you can even use it to melt ice on your pathway as shown below:
Add 1/4 cup of alcohol to two liters of water in a spray bottle. Add 10 drops of dish soap and mix. Spray your windshield when you get up in the morning and your car should be ready to go when you are.
The alcohol melts the ice and the dish soap lubricates the surface so it slides off easily.
Vinegar

Pure white vinegar or cleaning vinegar also has a lower freezing point than water which means vinegar can be used to melt ice. As vinegar is incredibly acidic, it should be diluted before use.
Mix equal parts hot water and vinegar in a spray bottle. Spray your windshield and windows in the evening to help prevent ice build-up overnight. You can also use it to help loosen ice in the morning.
Adding wood ash can also help prevent refreezing. This is an eco-friendly and rust-proof alternative to salt.
You can also combine vinegar with other solutions on this list. Sprinkle some baking soda on your step and pour vinegar over top or pre-mix a brine with salt for even application on driveways and walkways.
The downside to vinegar is it's acidity as well as the fact that it doesn't work very well in extremely low temperatures.
Beet Juice
Environmental experts in Canada have been exploring beet juice as an eco-friendly alternative to road salt. The brine contains salt, vinegar, and sugar to disrupt the ice. The molecular composition minimizes run-off so the brine stays on the road.
Your curious pet won't get sick from licking up some beet juice as the salt is diluted. However, their paws may get stained a bright purple.
Experts are also exploring cheese brine and pickle brine, though the smells are a limiting factor.
Hot Water
Using hot water alone will help melt ice in an emergency. However, it will quickly refreeze if temperatures outside are cold and could cause more harm than good. Adding vinegar or alcohol will minimize the risk of refreezing.
Choose Salt over Sugar
While you can use sugar to melt ice, salt works faster due to its molecular structure. However, sugar is a more environmentally-friendly choice and can be a good option in some circumstances.





