When winter rolls around the snow fall and storms cause ice to freeze on your concrete a lot of people run out and purchase rock salt or other deicers in order to melt the ice on their driveways and paths.
But you may have heard warnings saying that you shouldn't use salt on your concrete and this can actually lead to significant damage to your concrete. Is this true and why exactly is this the case?
All major salt deicers damage concrete by exacerbating the freeze/thaw cycle placing more pressure on the concrete as ice freezes, thus damaging the concrete. They also cause chemical reactions in the concrete which deteriorate it over time and they can even cause corrosion in the metal rebar inside the concrete.
New concrete that is experiencing its first winter is even more susceptible to deice damage as the concrete has generally not have enough time to cure.
However, most modern concrete is now fairly resistant to damage by deicers through a process called air entrainment. This is where bubbles are introduced into the concrete to create cavities too allow the ice to expand without damaging the concrete.
Some deicers are better on concrete than others and the amount of deicer you use also plays a big role in how badly your concrete is effected. It's important to know which products are the best to use so you can keep your driveways safe during the winter without damaging them.
Read on to learn more.
Do Salt Deicers Actually Damage Concrete As People Suggest?

Sometimes there are warnings out there based on speculation or old information but this isn't the case with deicers and concrete. Due to its major impact on infrastructure salt usage and concrete damage is fairly well researched leading to a fairly definitive understanding.
Yes, all salt deicers damage concrete in one way or another and damage concrete more than just regular water/ice does. Different deicers damage concrete in different ways and some are worse than others.
The best research paper I found on the matter is Effects of Deicers on Concrete Deterioration by David Darwin, JoAnn Browning, Lien Gong, and Sean R. Hughes.
They came to the following conclusions: (Note: NaCl = Sodium Chloride (Rock Salt), CaCl2 = Calcium Chloride, MgCl2 = Magnesium Chloride, CMA = Calcium Magnesium Acetate)
- At lower concentrations, NaCl and CaCl2 have a relatively small negative impact on the properties of concrete. At high concentrations, NaCl has a greater but still relatively small negative effect;
- At low concentrations, MgCl2 and CMA can cause measurable damage to concrete;
- At high concentrations, CaCl2, MgCl2, and CMA cause significant changes in concrete that result in loss of material and a reduction in stiffness and strength; and
- The application of significant quantities of CaCl2, MgCl2, and CMA over the life of a structure or pavement will negatively impact the long-term durability of concrete.
NOTE: Above is an excerpt from the paper Effects of Deicers on Concrete Deterioration by David Darwin, JoAnn Browning, Lien Gong, and Sean R. Hughes.
The question isn't really “do deicers damage concrete?” as it seems they all do to some extent.
The question instead becomes “how can we experience the least damage to the concrete that makes it a worthwhile tradeoff for having a safer surface?”
Sometimes it's worth a little bit of damage to have a safe driveway, footpath or road in order to make those surfaces safe to drive or walk on and to avoid injuries. Especially if the damage takes 20+ years to actually show the difference.
So now lets dive in and look at how exactly deicers damage concrete so you can understand exactly how this happens and you can work to avoid it.
The 3 Ways Deicers Damage Concrete
There are 3 major ways that deicers cause damage to your concrete.
1. Exacerbation Of The Freeze/Thaw Scaling
One of the biggest ways deicers damage concrete is through the exacerbation of the freeze/thaw scaling.
Concrete is a porous material and as a result it will absorb some water, especially into its upper layers.
When the water inside concrete freezes it expands in size and this expanding material places pressure on the concrete.
Concrete can actually withstand the pressure from frozen freshwater fairly well without significant damage but as soon as a salt deicer is added into the mix the problem gets worse for 2 main reasons:
A. Greater Expansion Of Ice When Freezing Placing Increase Pressure on the Concrete
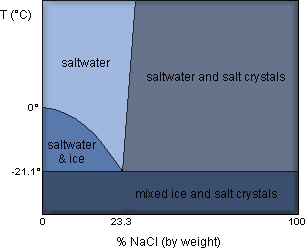
Salt water, when frozen, tends to be less dense than fresh water. This means that a salt water brine solution will cause MORE expansion than regular water when it freezes and this puts more pressure on the concrete leading to a higher chance of damage.
You might think that when salty water freezes you get a uniformly mixed salt and ice crystal to form, but this isn't what happens. Instead it goes through this process (explained by Frostburg):
- Ice crystals begin to form incorporating the water but leaving behind the salt (making the remaining liquid solutions saltier)
- The process continues with ice crystals forming leaving more and more salt behind – creating a concentrated brine in pockets of the concrete
- Finally flat ice crystals grow together trapping small pockets of concentrated brine and a cloudy, brittle frozen slush forms
- This mixture tends to be quite porous leading to less density and thus increased expansion during freezing – placing more stress on the concrete.
The density of pure ice is about 0.92 g/mL. The bubbles and pores in salty ice can make its density significantly less dense than pure ice (perhaps 0.8-0.9 g/mL).
This increased expansion is enough that the pressure it places on the concrete is too large for the concrete to handle – leading to damages.
The higher the concentration of deicer in the water the worse this issue becomes.
The reason you seen to see scaling (a flaking surface of the concrete) and spalling (hole or pockets in the concrete) near the surface of the concrete is because the highest concentration of water and brine soaks into the upper layers of the concrete.
It's harder for the water/brine mixture to soak all the way down into the concrete so the surface is the area mostly affected.
Modern concretes are designed to handle this increased pressure through air entrainment – where tiny air bubbles are introduced into the concrete to allow for more room for ice to expand into. This places less pressure on the concrete and means it is more difficult to damage.
However, they aren't perfect and are still susceptible to damages especially with high concentrations of salt brine or multiple freeze/thaw cycles.
Ice melts are also hydroscopic, meaning they have a tendency to absorb water from their surroundings and this can lead to a great quantity of water seeping into your concrete exacerbating the freeze/thaw cycle.
If you've ever had your ice melt bag leak liquid this is because the ice melt absorbs water from the air. The same process can happen inside or on top of your concrete.
B. More Freeze Thaw Cycles

Each time ice freezes and melt it expands and then contracts.
If ice has expanded and frozen once and then stays frozen it doesn't add as much stress to your concrete as it would if it freezes then melt then freezes then melts (and on and on).
One of the reasons deicers can damage concrete is they make the freeze/thaw cycle happen more frequently than they otherwise would.
When a storm rolls in and temperatures drop ice freezes on (and in) your concrete. During a cold spell that ice could stay frozen for days, weeks or months at a time depending on where you live.
However, if you apply salt to the ice then the salt will lower the melting point of ice and turn it into liquid. This will usually happen during the day.
Then when night time rolls around the temperatures could drop so low that the ice now freezes again. During the day it might warm up enough to melt the salt water mixture (or you might apply more salt deicer) and the process repeats multiple times through the winter season.
This repetition of the freeze/thaw cycle places must more stress on your concrete and is more likely to lead to damage.
Magnesium Chloride Is An Exception To This Freeze/Thaw Cycle Issue
An interesting exception to this freeze/thaw cycle issue is the deicer magnesium chloride.
Magnesium chloride and calcium magnesium acetate seem to have a unique reaction with the concrete that prevent some of the stress from the freeze thaw cycle.
Concrete is alkaline (which means it has a Basic pH level) and when exposed to an alkaline environment magnesium will react and form an insoluble precipitate called magnesium hydroxide.
What this means is the magnesium hydroxide likely plugs up the holes on the surface of the concrete. This provides a protective barrier to protect the concrete from soaking up as much water/brine.
However, this does NOT mean you should run out and buy magnesium based deicers and they are actually cause quite a lot of damage to concrete through a chemical reaction with the concrete itself.
2. Direct Chemical Attack

All of the common deicers will cause chemical reactions with concrete than can cause damage over time. There is no common deicer that doesn't do this.
Each chemical will have different reactions with the concrete and while this is out of the scope of this particular article (and extends beyond my research) what I can tell you is which type of salts are the LEAST damaging to concrete (chemical wise):
- Least damaging: Plain Salt (Sodium Chloride)
- Next least damaging: Calcium Chloride
- Most damaging: Magnesium Chloride
It's interesting to see magnesium chloride as the most damaging chemical wise as that was the least damaging when it came to the freeze/thaw cycle.
While this may have you scared that all deicers will ruin your concrete there have actually been field studies (where they look at real world examples of deice use and its effects on concrete) and they found that calcium chloride seems to have negligible effects on concrete durability and the effects of magnesium chloride appear to be slow.
Studies of “Salt” Scaling of Concrete by Portland Cement Association came up with these interesting findings:
- Relatively low concentrations (of the order of 2 to 4 percent by weight) of de-icer produce more surface scaling than higher concentrations or the absence of de-icer.
- On the basis of the foregoing, it appears that the mechanism of surface scaling is primarily physical rather than chemical.
- Surface scale test procedures greatly influence the rate of scaling. The most severe test procedure yet discovered is one in which the concrete is alternately frozen and thawed with the de-icer solution remaining on the top surface of the concrete rather than being replaced with fresh water prior to each freezing.
- No scaling was produced when the concrete surface had no free water on it during the freeze portion of the cycle.
- A period of air drying of the concretes prior to the start of scaling tests increased the resistance to surface scaling. Air-entrained concretes treated in this manner were immune to the most severe scale test procedure for more than 200 cycles of test.
3. Rebar Corrosion
Steel rebar is used in concrete to provide reinforcement against tensile stress making the concrete stronger overall.
However, corrosion of embedded steel rebar is the most common form of concrete deterioration and deicing can cause the steel rebar in your concrete to corrode faster.
Most of us know that salt can cause metal to rust. You might see deterioration of your vehicles during winter months when they are exposed to salt or if you live anywhere near the ocean (like I do at the moment) then the salt water in the air will wreak havoc on anything metal.
When iron corrodes it turns into iron oxide (rust). This causes it to expand and puts pressure on the concrete just like our freezing water does.
Because the iron oxide has nowhere to expand into this can cause cracking in the concrete and the deterioration of the rebar also causes your concrete to lose the strong structure the rebar provides.
Salt water causes iron to corrode into iron oxide faster than just regular water would.
Cracks in the concrete can also lead to moisture more easily getting the the rebar speeding up the corrosion process even further.
Modern Concrete Is Resistant To Damage By Deicers

Modern concrete is now made to be fairly resistant to the common damage caused by deicers. They do this through a process called air entrainment.
This is a process where air bubbles or small voids are created in the concrete. This creates cavities in the concrete which provides space for water (and salt water) to expand into ice without exerting an much pressure on the concrete.
This leads to less cracking, scaling, spalling and exposure of the aggregate and means your concrete will last longer than older concrete would.
However, concrete ranges in quality and it can be difficult to tell whether or not your concrete is up to modern standards. Companies can cut corners or there can be issues in the manufacturing or pouring of concrete that can cause quality issues with the final product.
New Concrete Is More Susceptible To Damage From Deicers

However, even with modern concrete you do need to wait for the concrete to completely harden and cure.
New concrete is particularly susceptible to damage from deicers, especially during their first winter.
While concrete tends to harden and cure enough to support vehicles between 2-7 days after pouring, and while many people assume concrete is fully hardened after 28 days or so the truth is concrete will continue to cure and harden over its entire lifespan.
This means concrete that is 10 years old will still be curing and hardening (though likely at a much much slower pace).
For this reason it is suggested that you completely avoid using deicers on concrete during it's first year and you should ideally let a full winter pass without using any deicers. If you want to be sure it's even better to wait 2 winters before using deicers on your concrete.
This gives the concrete long enough to harden so chances of cracking, scaling and spalling are significantly reduced.
Instead of using deicers you should consider using abrasive materials that add traction to the top of the ice without actually melting it.
Sand does not melt ice but will provide traction for foot traffic was well as cars and kitty litter also won't melt ice but will provide traction similar to sand. Just make sure to buy the correct kitty litter for ice as some forms are much better than others for traction.
How To Minimize The Impact Of Deicers On Your Concrete

Sometimes you need to use ice melt on your driveway due to the fact that the ice is a safety hazard.
When using ice melt there are a few things you can do to minimize the negative impact it might have on your concrete.
Choose The Best Ice Melt For Concrete


The type of ice melt you choose has a major impact on how much damage your concrete is going to experience.
From my research it seems like calcium chloride, when used in small amounts, is the least detrimental to your concrete with sodium chloride (regular rock salt) having a similar effect.
Magnesium Chloride or Calcium Magnesium Acetate (CMA) seems to be one of the worst options to choose for concrete.
Use Small Amounts Of Ice Melt




It seems that the more deicer you use the larger the detrimental effect it has on your concrete.
Above you can see results of the experiment where they use smaller concentrations of deicer vs higher concentrations and the MASSIVE differences it had on the concrete.
Calcium Chloride in particular seemed more susceptible to this increased damage when used in high quantities (as shown by the 95 vs 10 week comparison) whereas regular rock salt still showed more damage but not to the same extreme.
When using a deicer it seems you should use as little as possible to get the job done and no more.
To melt ice with salt as effective as possible prepare the area first by scraping or shoveling away as much snow and ice as possible.
Then choose the best type of ice melt for your driveway (and climate) and spread it lightly and evenly over the ice. For a more even spread you can even create a brine solution to spray over the ice.
Click here to learn how to apply ice melt for the best results.
Once Melted Wash Away The Ice Melt Using A Hose

Ice melt usually only takes 20-30 minutes to start working and most of the ice is gone fairly quickly, especially in warmer weather.
It is advised that once the ice melt has done it's job and your driveway or pavement is freeze from ice you should wash it thoroughly with a hose.
Hosing off your concrete will wash away a lot of the salt brine, lowering the concentration of brine and protecting your driveway from the damage potentials mentioned above.
Use Sand or Other Abrasive Materials Instead of Traditional Ice Melt

There are a variety of methods to remove ice from a driveway without salt and you can try a variety of these if you want, but all deicers have similar problems when it comes to concrete.
Another good alternative is to use sand, clay, kitty litter or some other abrasive material to provide traction on top of the ice.
This won't melt the ice but it'll reduce the chances of slipping happening.
For best results use wet sand (so the sand doesn't blow away) or you can even mix sand with a low concentration of ice melt to help the sand melt into and stick in the ice.
This won't melt the ice much, nor damage your concrete much, but it'll stop the sand from freezing and help to provide better traction, especially in very cold climates.
Resources
Take everything I say with a grain of salt (pun intended haha) and always look to the research yourself when making decisions.
Here are some of the helpful resources and academic papers I used when doing research for this article:
105-M70 – Effects of Deicers on Concrete Deterioration by David Darwin, JoAnn Browning, Lien Gong, and Sean R. Hughes
302.1R-15 Guide to Concrete Floor and Slab Construction (Reported by ACI Committee 302)
Avoid Salting The First Winter by Concrete Construction
Studies of “Salt” Scaling of Concrete by Portland Cement Association




