There is a lot of confusion about how salt and ice interact. Salt is often used to melt ice during the winter, but it's also used to keep the contents of a cooler cold for longer.
This confusion leads many to ask if salt makes ice colder, and if so, why? How exactly does this work?
Salt makes ice colder in 3 ways.
- As salt dissolves into water it uses energy making it colder
- Salt lowers the melting point of ice causing the ice to melt. This melting process requires energy (in the form of heat) and this makes the remaining ice (and water) colder.
- Due to it's lower melting point partially melted salt water will stay at a lower temperature (around 16-28ºF/-2 to -9ºC) than partially melted fresh water (32ºF/0ºC).
Still confused? Not to worry; let's dive into the science and learn how salt makes ice colder.
The 3 Ways Salt Makes Ice Colder
1. As Salt Is Dissolved Into Water It Makes That Water Colder

This is a small part of the cooling effect salt has on ice, but it's the first step and important to understand.
You may not see it but on the surface of every ice cube (or sheet of ice) is a thin layer of water that is in a constant state of melting and refreezing.
When you put salt on top of ice what happens first is that the salt begins to dissolve into the layer of water on top of the ice.
Below you can see a video animation of how water dissolves salt:
Here's how dissolving salt makes ice colder…
For salt to dissolve into water energy (in the form of heat) is required to make this chemical process happen.
This energy is taken from the surroundings (the existing ice) and this makes the ice colder than it would have been otherwise.
This is called an “endothermic reaction” – meaning it absorbs heat and lowers the temperature of the surrounding area creating a cooling effect.
You can see this process in action in the below video where dissolving salt into water lowers the temperature of the water from 65.3ºF/18.5ºC down to 62.6ºF/17ºC.
That's a drop of 2.7ºF/1.5ºC just by dissolving salt into water!
You can also see this process in a more extreme way in instant cold packs. When you use instant cold packs a chemical (eg. urea) is dissolved into water. This process is very endothermic so it takes heat from the water making the ice pack cold.

So as the salt dissolves into the water on top of the ice a small cooling effect takes place. But this is not the major reason salt cools ice.
That comes down to the melting process.
2. Salt Causes Ice To Melt Which Makes The Remaining Ice Colder
The biggest reason ice gets colder when you add salt is that salt causes ice to melt.
See when water freezes it forms a crystalline structure. When salt mixes with the ice it interrupts the bonds and makes it more difficult for water to stay in solid form.
The causes what is known as “freezing point depression”.
In simple terms: the freezing point of the water is now lower than it was previously.
While freshwater ice freezes at 32ºF/0ºC saltwater ice requires a lower temperature to freeze. Usually around 16-28ºF/-2 to -9ºC depending on how much salt is used.
When you pour salt on top of ice it creates this freezing point depression and this causes the ice to begin to melt at a rapid pace.

Now here is how this makes ice colder.
Remember how dissolving salt in water was an endothermic reaction? Aka it absorbs heat and lowers the surrounding area?
Well melting ice is also an endothermic reaction.
It takes energy (in the form of heat) for ice to melt into water. It takes this heat from the surrounding ice making that ice colder!
So because the salt makes the ice melt quickly, a lot of heat is required to make this melting happen.
That heat energy is sucked out of the ice making the ice colder in the process.
And now due to freezing point depression the ice will stay colder.
3. Partially Melted Saltwater Stays Colder Than Partially Melted Freshwater

We talked about how adding salt to ice actively makes that ice colder because dissolving and melting are both endothermic reactions.
But also as saltwater ice begins to melt it was stay colder than freshwater ice as it melts.
Did you know that when you boil water on a stove the water never goes above 232ºF/100ºC?
Even though the stove itself can be as hot as 1,112ºF/600ºC for an electric stove or up to 3,542ºF/1,950ºC for the flame on a gas stove.
But wait, why doesn't the water get as hot as the stove? And what does this have to do with ice?

This will all make sense in a second.
See when you boil water the water will get up to 232ºF/100ºC then any extra heat energy you add doesn't go into making the water hotter, but rather converting the water from a liquid state into a gas.
The water will stay at 232ºF/100ºC until it has all been turning into a gas. After which your metal pot can get extremely hot!
Now how does this apply to salt making ice colder?
Well the same process happens as ice melts and turns from a solid into a liquid.
For freshwater ice its melting point is 32ºF/0ºC. When it reaches this temperature it will not get hotter, but rather any extra heat energy is used to convert the water from a solid into its liquid form.
So a mixture of ice and water will stay at around 32ºF/0ºC until all the ice is gone and then it will begin to warm up.
However, when you add salt to the ice it causes freezing point depression. The new freezing point of the saltwater ice is now 28ºF/-2ºC to 16ºF/-9ºC depending on how much salt is in the water.
Let's work with 20ºF/-7ºC for now.
This means when the temperature reaches 20ºF/-7ºC the saltwater ice will begin to melt. It also means that any heat energy you add to this partially melted ice WILL NOT INCREASING ITS TEMPERATURE.
The heat energy will go into converting the saltwater from solid ice into liquid water. Once all the ice is melted then the saltwater will begin to warm up.
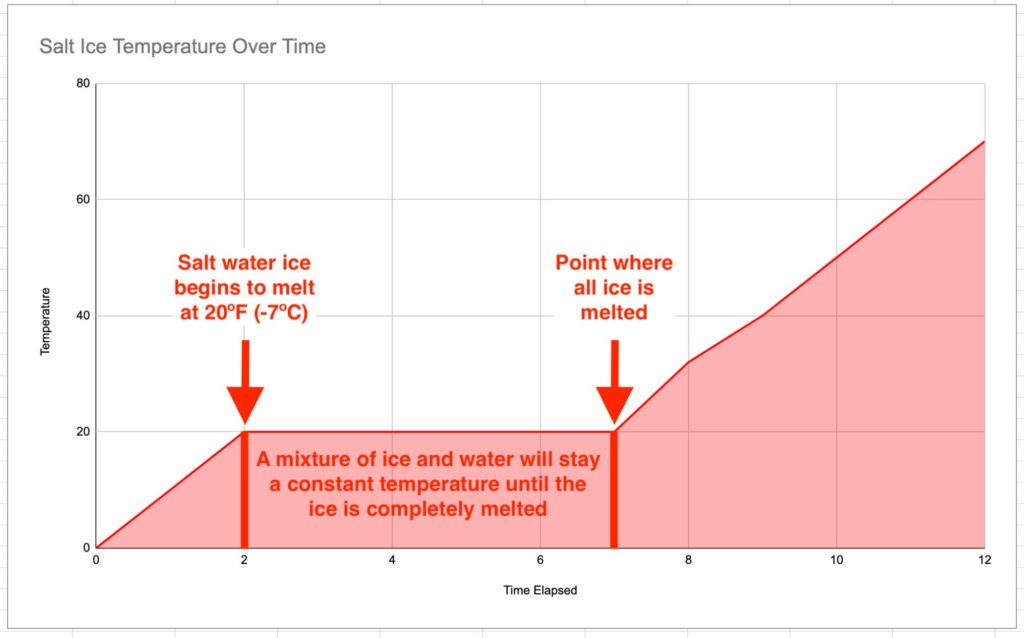
This means that partially melted saltwater ice will stay colder than partially melted freshwater ice.
How Cold Exactly Does Salt Make Ice?
The exact temperature of the ice after salt is applied will ultimately depend on the starting temperature, outside temperature, amount of salt used and how quickly that salt dissolves into and melts the ice. With a lot of salt it's possible to get ice as cold as 0ºF/-18ºC.
This is why salt is used in ice for making ice cream. The ice on the outside of the churner has salt added to it. It can go as low as 0ºF/-18ºC (the same temperature as a household freezer) and this will then freeze the ice cream.
The starting temperature of ice will play a role in how cold the ice gets when salt is added.
For example, ice frozen in the average home freezer will start at 0°F/-18° C. Conversely, ice frozen outside in the winter (eg. on the road or your driveway) could be as warm as 30°F/-1°C, depending on the weather.
The starting temperature of the ice can play a major role in how cold salt will make the ice. Outside temperature also plays a major role as the faster the salt melts the ice the more energy is absorbed and the colder the ice will get.
Salt can cause ice to get extremely cold. In the below experiment salt made the ice as cold as 1ºF/-17ºC.
The biggest difference to note is between the temperatures of melting ice in saltwater versus melting ice in freshwater.
As freshwater melts, the ice continues to warm up until it reaches a liquid state. When the water temperature hits the freezing point of 32ºF (0ºC), it will stay there until the ice is melted.
As saltwater melts, the ice and surrounding water become colder than regular ice during the partially melted phase of the process.
When the temperature of saltwater ice hits the new freezing point, the water and ice will remain at that temperature until all of the ice is melted.
This keeps the temperature of the saltwater ice and meltwater at subzero temperatures.
Is Frozen Salt Water Colder Than Frozen Fresh Water?

With all of this fancy science in mind, it's natural to think that frozen saltwater must be colder than frozen fresh water, right?
Not necessarily.
Both saltwater and freshwater will only freeze as cold as the surrounding temperature. If you put a saltwater and freshwater ice cube in the freezer, they'll both freeze to 0° F/-18° C.
The difference is when the liquids start to melt.
Freshwater will start to melt when the water temperature reaches 32ºF/0ºC. Saltwater will start to melt when the water temperature reaches to 16ºF/-9ºC
So, if you set a timer and open your freezer to check the ice cubes, you'll see the freshwater start to freeze before the saltwater.
However, if you measure the ice temperature after both are completely solid, they'll be the same.




