If you live in an area with ice and snow, you're likely used to throwing salt on walkways and roads during the winter. While it's well-known that salt makes ice melt faster, many are unaware of the science behind the process.
Salt lowers the freezing point of water from 32ºF (0ºC) to as low as 16ºF (-9ºC ). As a result, ice starts to melt at a lower temperature when salt is present. Salt sprinkled on ice starts melting the thin layer of water on top, prevents it from re-freezing and begins to melt the rest of the ice.
Here's what you need to know about why salt makes ice melt faster, and how you can keep your walkways clear this winter.
Why Does Salt Make Ice Melt Faster?
Salt lowers the freezing point of water from 32ºF (0ºC) to 16ºF (-9ºC) in a process called “freezing point depression.” This means it must be colder than usual for the water to freeze into ice.
Even when ice appears frozen solid, there's a thin layer of water on top— often imperceptible to the human eye. This layer will continually unfreeze and refreeze.

The salt mixed with this thin layer of water and prevents this water from re-freezing.
The process continues with new layers of water on the ice until it melts completely.

The lower freezing point also means the water requires a lower temperature to remain frozen.
If you were to place a regular ice cube in 32ºF (0ºC), it would retain its form. If you were to add salt, the ice would start to melt despite the temperature being at the usual freezing point.
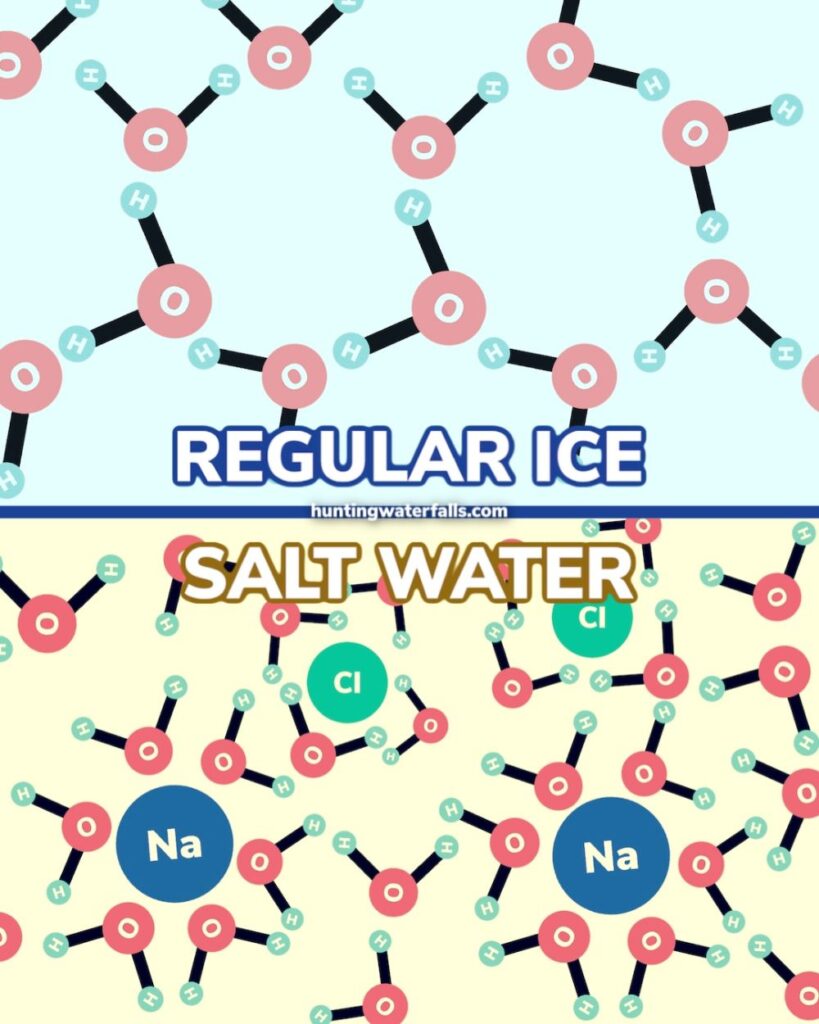
The reason this happens is the salt molecules get in the way of the water molecules and stops them from forming the strong bonds needed to turn into solid ice.
The freezing point is still a limiting factor. If you live in a place that gets below 16ºF (-9ºC), normal salt won't work as the temperature is colder than the freezing point.
There are a few types of ice melt designed to work at colder temperatures, though they're more expensive than generic road salt. See the full list of ice melt alternatives.
Why Does Ice Melt Faster In Freshwater Compared To Salt Water?
While salt will melt ice faster, ice can stay frozen longer in salt water compared to fresh water. This seems counterintuitive given salt lowers the melting temperature of ice but the reason behind this is quite interesting.
The science behind this phenomenon is all about density and displacement.
Cold water is denser than warm water. Therefore, when ice is placed in fresh water, the cold water from the melting ice sinks, forcing the warm water up. The heat from the warm water then begins to melt the ice even more – forcing cold water down and more warm water up.
The cycle continues until the ice is melted.
This process is called convection and is one of the ways that heat can be transferred.
The same thing happens with air. Warm air rises, and cold air sinks. That's why we use ceiling fans in different directions each season to circulate the air.
As the cold fresh water moves away from the ice cube and the warm water moves up, it causes the ice cube to melt faster.
Saltwater is denser than fresh water. So when the ice cube starts to melt, the cold fresh water sits on top of the salt water, keeping the ice colder for longer.
The cold water is unable to sink to the bottom and the warm salt water is unable to force itself up to come in contact with the ice cube. So the ice cube is protected by a layer of melted water.
Salt water stops convection from happening so the fresh water ice will last longer in salt water.
You can see this in action in the video below:
NOTE: If you had a salt water ice cube placed on top of salt water then the convection would happen. It's the difference density of the fresh water ice cube compared to the salt water it sits in that causes this phenomenon.
This scientific process is the same reason icebergs last so long in salt water compared to fresh water. It's also why ice will cool a diet soda faster than a full-sugar soda.
You can see the difference in soda density by placing a can of diet soda and a can of regular soda in a tub of water. One will float immediately (diet), and the other will float slower (regular) because of the difference in density due to the amount of sugar used.
So, while salt will melt ice faster, salt water will not if (and only if) the fresh water ice is floating on top of the salt water.
Pouring salt water onto ice WILL make that ice cube melt faster.
Is Salt The Fastest Way To Melt Ice? What Melts Ice Faster Than Salt?
Salt is one of the fastest and most effective ways to melt ice but it isn't the only way. Alcohol and a variety of other chemicals can also be used to melt ice quickly.
Salt is commonly used because it is relatively safe to use, widely available and extremely affordable and easy to spread.
There are several types of salt ice melts to choose from depending on your needs.
Sodium chloride (rock salt) is often used due to its affordability and immediate traction. Some places use salt brines or beet brine as an alternative to loose salt.
Ice melt blends of magnesium chloride or calcium chloride are more effective than true salt in extreme temperatures. Plus calcium chloride can be less damaging to concrete compared to some other ice melts, making it a popular choice.
Magnesium chloride at temperatures as low as 5ºF (-15ºC). This ice melt option is less harmful to plant life and pets, making it a safe alternative to salt. However, magnesium chloride is one of the worst offenders when it comes to chemical damage to concrete so sometimes it should be avoided.
Calcium chloride is an inorganic salt compound. It works at temperatures as low as -25ºF (-31ºC) and works faster than salt.
Using heat mats or hot water can also melt ice faster. Sprinkling salt over ice build-up, then pouring hot water over top can be very effective when you're in a rush.
Alternative using sand to add traction over ice won't melt the ice but it'll instantly make it safer to walk and drive on.
Both magnesium chloride and calcium chloride are more expensive than rock salt, which is why most transportation departments don't use them. However, these are viable options if you're looking for something to keep your driveway and walkway clear.
Does Salt Stop Water From Freezing?
There's a common misconception that salt water won't freeze at all. Many people use the ocean as an example.
Salt doesn't stop water from freezing, but it can disrupt the freezing process.
Salt molecules block water molecules from binding into ice. Yet, it's freezing point depression, not freezing point prevention.
It means that temperatures need to get colder in order for the water to freeze into ice. Rather than temperatures needing to be below 32ºF (0ºC) they might need to get as low as 16ºF (-9ºC) for the salt water to turn into ice.
The ocean does freeze, despite being salt water— that's where icebergs and the polar ice caps come from. If you took a cup of ocean water home and put it in the freezer, it would turn into ice.
This is because your home freezer is usually set to 0ºF (-18ºC) which is colder than the freezing temperature of salt water.
Interestingly, as your salt water ice melts it'll stay at 16ºF (-9ºC) until all the ice is melted. Because salt ice is colder than regular ice it can be used in coolers to keep items frozen. If you need to keep meat frozen using salt ice or ice packs can be a great solution.
Other factors prevent the ocean from freezing solid in subzero temperatures include volume, pressure, currents, and geothermal heating. Consider the fact that the temperature at the bottom of Mariana's Trench is 34-39°F (1-4ºC).
Does Salt Make Water Harder To Freeze?
Salt does make it more challenging for the water to freeze.
Say you sprinkle road salt on the ice on your doorstep. The temperature gets to just above freezing, and the ice starts to melt.
Then the sun goes down, and the temperature drops to 28ºF (-2ºC). The salt molecules disrupt the freezing process.
The partially melted ice won't refreeze into a solid. It will likely remain as a slush until the temperature drops further.
Once the temperature drops to around 16ºF (-9ºC) then you'll notice that yes the salt water on your doorstep will begin to full freeze over.
Will Water Freeze With Salt?
Water will still freeze when it reaches its modified freezing point.
When regular salt is added, the water will freeze at 16ºF (-9ºC). This can be slightly higher or lower depending on the quantity of salt in the water and what type of salt it is.
As the standard home freezer temperature is 0°F (-18°C), salt water will freeze— it will just take longer.
However, water mixed with sodium chloride (which is also a salt) won't freeze until temperatures as low as -25ºF (-32ºC). So this water with salt in it won't freeze in your freezer at home.
Choose Salt This Winter
Salt remains the most accessible, effective way to melt ice if you live in a cold climate. If you live in an area that gets colder than 16ºF (-9ºC), consider magnesium chloride or calcium chloride.




