There's a lot of confusion about the effect salt has on ice. You've likely heard that adding salt to your cooler will make the ice melt slower. You've probably also heard that adding salt to icy paths and roads will make them thaw faster.
Salt can make ice melt slower when freshwater ice is floating in salt water. As saltwater is denser than the melting ice, the cold, fresh water has nowhere to go. The dense salt water keeps the cold fresh water around the remaining ice for insulation.
Compare this to freshwater ice floating in freshwater. As the ice melts the cold water will sink to the bottom which will push warmer water up towards the ice causing it to melt faster.
This transfer of heat is know as convection and because saltwater is denser than freshwater it can stop this convection from happening.
Salt can both make ice melt faster and slower, depending on the situation. Let's explore the science that makes this possible.
When Will Salt Make Ice Melt Slower
Salt will make ice melt slower when ice is floating in salt water, due to the density and displacement.
Ice melts in liquid due to heat transfer. Essentially, the ice absorbs the heat from the surrounding water and that heat energy is used to convert the water from a solid into a liquid.
Freshwater ice must stay at 32ºF (0ºC) to stay frozen. As the ice “absorbs” the surrounding temperature, the bonded ice molecules start to move faster and transition from a solid to a liquid.
Cold water is denser than warm water. You have have heard the phrase “heat rises” and the opposite is also true – “cold sinks”.
As the ice melts and releases cold water into the surrounding area, that cold water starts to sink.
As the cold water sinks, it displaces the warmer water and forces it upward. As the warm water moves up and surrounds the ice, it causes the ice to melt faster. Which continues the process.
Saltwater is denser than fresh water. When the ice starts melting, the cold water floats on top of the salt water.
The salt water is still denser than the cold melted water from the ice so the freshwater doesn't go anywhere.
This means the existing ice is now sitting in (and insulated by) the cold water that has just melted.
Because there's less available heat close by for the ice to absorb it melts slower. So in this way saltwater makes the ice melt slower.
This is why icebergs last so long in salt water compared to fresh water.
Below you can see this in action in a simple science experiment you can do yourself.
When Will Salt Make Ice Melt Faster

More often than not salt will NOT cause ice to melt slower. In fact the opposite is true. Salt usually makes ice melt faster than it otherwise would.
When salt is applied directly to ice— as opposed to ice floating in saltwater— the ice will melt faster.
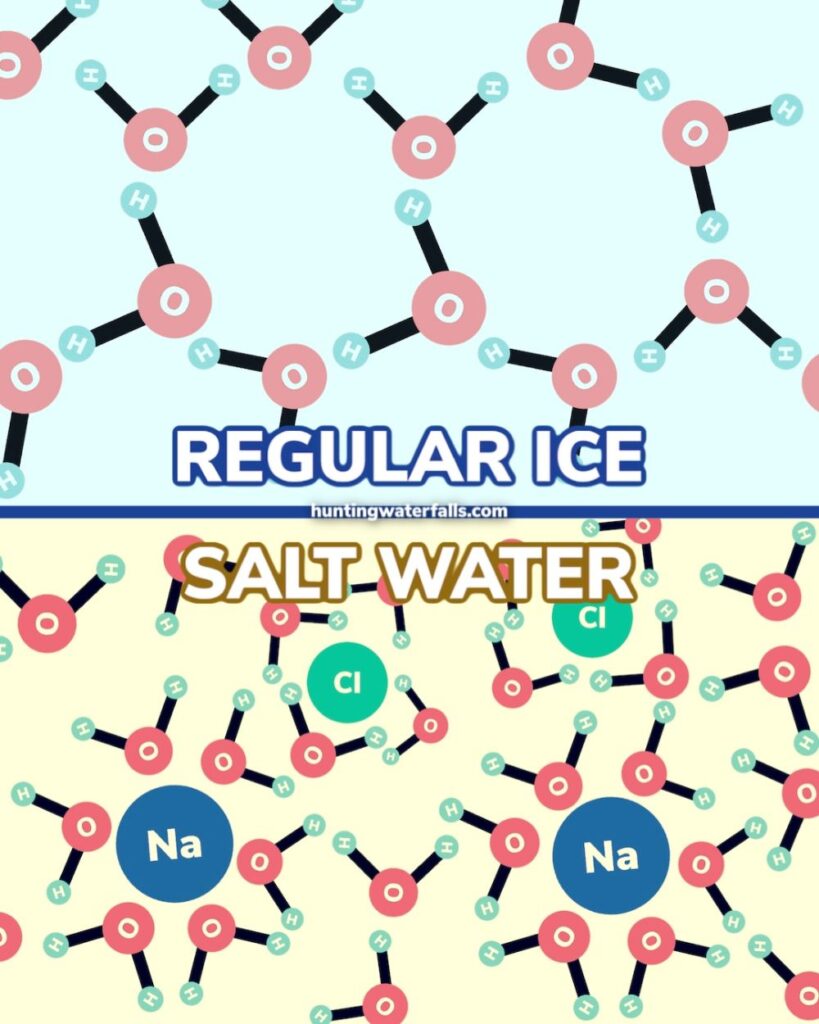
Salt lowers the freezing point of water from 32ºF (0ºC) to 16ºF (-9ºC). This is known as freezing point depression.
Freezing point depression is a chemical process known as a colligative property. In simple terms, salt changes the freezing temperature of ice and lowers it from 32ºF (0ºC) to as low as 16ºF (-9ºC).
So at an example temperature of 28ºF (-2ºC) regular freshwater ice would stay frozen. But when you add salt its freezing temperature is lowered to below that temperature and so it melts.
This is because the salt molecules interrupt the water molecules. The salt gets in the way and stops them forming solid bonds as easily.
When freezing point depression takes place, it must be colder than usual for the water to freeze into ice. It also means that the ice will start melting as soon as the temperature gets warmer than 16ºF (-9ºC).
Ice has a thin layer of water on top of the solid surface, which is usually imperceptible to the human eye. When you sprinkle salt on top, it prevents this water from freezing.
As the ice absorbs the heat from the liquid layer, it melts another layer of water underneath. The process continues with new layers of water on the ice until it melts completely.
If you were to place a block of ice outside at 32ºF (0ºC), it would retain its form. If you were to add salt, the ice would start melting unless the temperature dipped below 16ºF (-9ºC).

Does Salt Water Ice Melt Faster Or Slower Than Pure Water
Saltwater ice will melt sooner than freshwater ice, but this also causes it to stay colder than regular ice. This is why many camping experts recommend adding saltwater ice packs to your cooler.
This concept seems contradictory, but the science makes sense.
As the freshwater ice melts, the water quickly warms up to the freezing point at 32ºF (0ºC). This speeds up the melting process.
The saltwater ice starts melting faster but stays colder longer. All the partially melted ice will remain at 20-28ºF (-7 to -2ºC) until the ice is completely melted.
So, if you're melting equal cubes of saltwater and freshwater in the sun, the saltwater cube will melt faster. However, if you're adding ice to a cooler, the saltwater ice will keep the contents colder for longer.
Does Saltwater Freeze Colder than Freshwater
Both saltwater and freshwater will only reach the freezing temperature of their surroundings.
For example, the average household freezer is 0ºF (-18ºC). Water frozen in this freezer will never get colder than 0ºF (-18ºC), whether or not it has salt in it.
The difference is that saltwater will remain as a liquid for longer than freshwater. It will take longer to turn into it's frozen state.
It'll also melt earlier than freshwater once it's out of the freezer.
What Else Slows Down Ice From Melting
If the surrounding liquid is denser than the frozen water, the ice will melt slower. So, an ice cube placed in orange or grape juice will melt slower than an ice cube in water. This is one of the reasons restaurant ice seems to last so long.
This is the same displacement science that makes ice melt slower in saltwater. Orange and grape juice are denser than water because they contain a lot of sugar, so the melting ice has nowhere to go and sits on top of the juice.
Insulation also slows down the melting process. Using a vacuum-insulated bottle or foam-insulated cooler will help keep ice colder for longer.
Vacuum insulation prevents convection and minimizes heat transfer. This technology is also lighter than foam insulation products.
Insulated bottles and coolers keep the interior temperature colder for longer. The more time it takes for ice to reach its freezing or melting point, the longer it remains in its solid state.
There are actually lots of different ways to stop ice from melting or getting it to melt slower.
How to Make Ice Stay Frozen for Longer

There are a few things you can do if you're packing a cooler for a camping trip or hike and want your ice to stay frozen for as long as possible.
Start by pre-chilling your cooler overnight before your trip. If your cooler won't fit in a freezer, add a layer of ice and replace it when you pack.
Use ice blocks instead of crushed ice or cubes in your cooler. Less surface area will result in less heat transfer, keeping the blocks frozen for longer.
If you're trying to keep a water bottle cold, get a water bottle ice tray with long, rectangular blocks. These will offer the same benefit as an ice block and fit through the bottle opening.
Use a high ratio of ice to contents when packing a cooler and layer the contents, ideally 2:1. Consider frozen food products a type of ice.
Add Salt to Your Cooler
When used appropriately, salt can slow down the melting process. Adding saltwater to your cooler will cause the ice to start melting faster, but will make the cooler colder overall.




